
Tailored blood, at scale
Our mission is to make blood tailored for the people who need it, at scale & viable economics for broad civilian & military use
At Safi Biotherapeutics, we manufacture adult stem-cell derived, human red blood cells (mRBCs), with the goal of providing a highly characterized cell therapy blood transfusion product at industrial scale and viable economics for civilian and military transfusion needs. Blood is the most prescribed biologic therapy in the world. Blood donor supply shortages continue to be a global crisis, and we are uniquely positioned to provide a safe and reliable blood supply to meet this challenge. Our mRBCs have valuable potential use in chronic transfusion indications as well as in acute transfusion settings, such as hospitals and battlefields.
Modern & scalable biomanufacturing that meets purity & quality standards
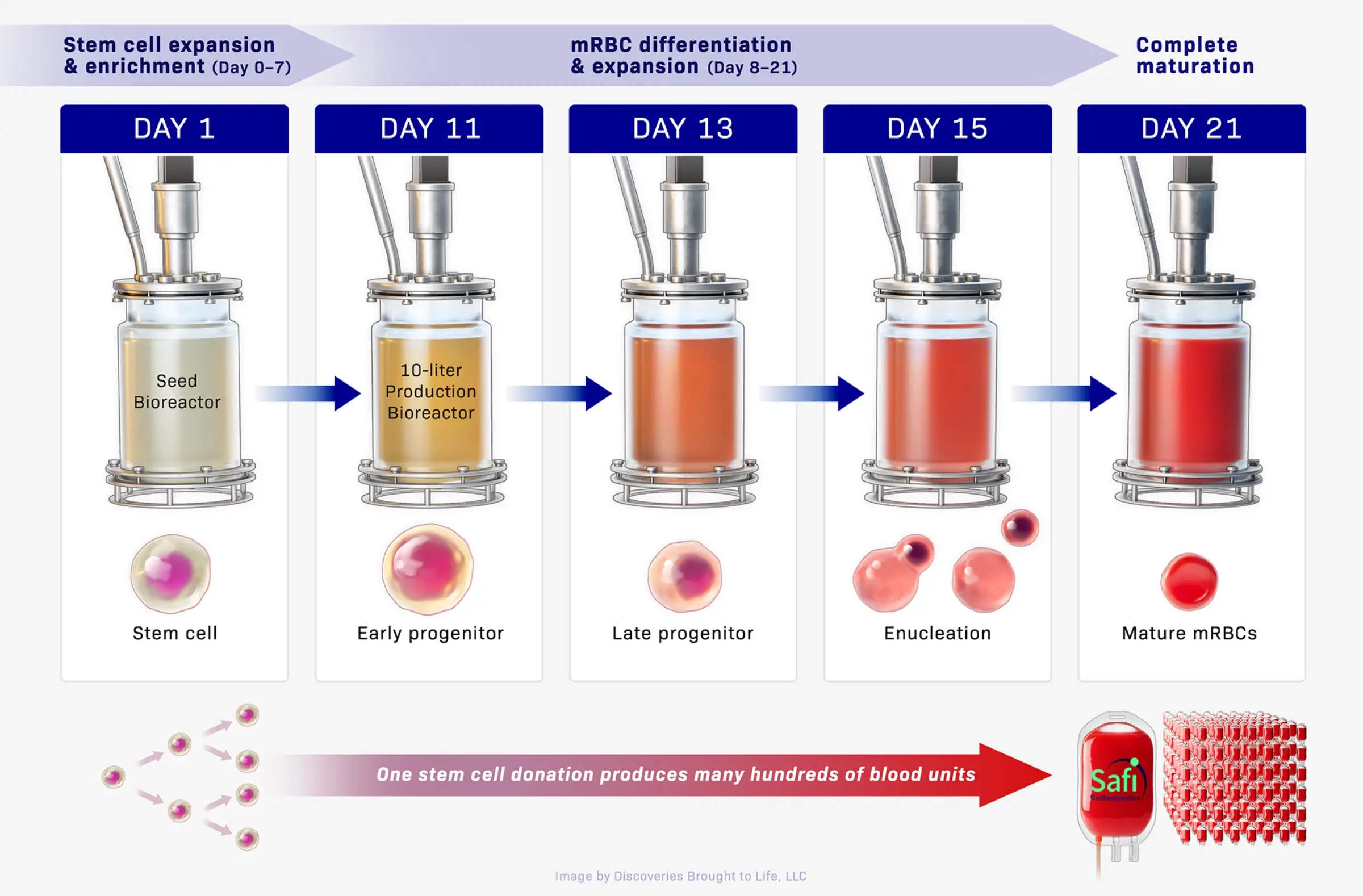
Safi’s manufacturing blueprint for red blood cell production is the most advanced in the industry. Our collaboration with ARMI | BioFabUSA, a non-profit organization and U.S. Department of War Manufacturing Innovation Institute, is supporting the GMP production of mRBCs for clinical trials. This collaboration, alongside our proprietary advanced biomanufacturing processes, enables the manufacture of units of ultra-high densities of mRBCs in 10-liter scale bioreactors that consistently meet strict purity and quality standards. GMP manufacturing for initial clinical studies at 10-liter scale is currently underway.
Advanced cryopreservation & stockpiling
We continue to advance our research of cryopreserved and freeze-dried mRBCs, which present innovative solutions across civilian and military settings, particularly with respect to pre-hospital use and government stockpiling. We have achieved proof of principle showing our mRBCs are more resistant to the detrimental effects of both cryopreservation (without glycerol) and freeze-drying. This early work provides a foundation for optimization of cell specification and formulation that maximizes product viability for long-term storage and transport.
About Safi
Founded in 2020 as the cell therapy commercialization partner of the U.S. Defense Health Agency On-Demand Blood program, Safi has received more than $20 million in non-dilutive government funding and completed a $5 million private seed investment. These investments are accelerating the GMP manufacturing of our red blood cells at clinically meaningful scale and the completion of IND-enabling activities for clinical trial initiation in 2027. In 2024, the U.S. Food and Drug Administration granted our mRBCs Rare Pediatric Disease Designation and Orphan Drug Designation for use in the chronic transfusion of patients with sickle cell disease.
Recent news featuring Safi Biotherapeutics
Safi Biotherapeutics and ARMI | BioFabUSA Complete Successful Technical Transfer of Initial Clinical Scale 10L mRBC Production System JAN. 8, 2026
Safi Biotherapeutics and Functional Fluidics Initiate Collaboration to Validate Health and Quality of Manufactured RBCs JUL. 16, 2025
Safi Biotherapeutics Awarded NIH STTR grant to Improve Blood Transfusion Safety APR. 24, 2025
U.S. DoD Awards Safi Biotherapeutics $3.5 Million in Additional Non-Dilutive Funding to Optimize Cryo-Storage of mRBCs FEB. 25, 2025
Safi Biotherapeutics and ARMI | BioFabUSA Initiate Collaboration to Support Large-Scale Development of mRBCs FEB. 10, 2025
Safi Biotherapeutics Granted Rare Pediatric Disease Designation and Orphan Drug Designation from the U.S. FDA DEC. 18, 2024
Safi Biotherapeutics Secures $5M in Seed Funding to Accelerate Plans to Grow Blood for Acute and Chronic Transfusions SEP. 11, 2024
This government-backed startup is growing blood to help fight shortages SEP. 11, 2024
Safi Biotherapeutics Solidifies Leading Position in Red Blood Cell Biomanufacturing with Acquisition of EryPharm Assets JAN. 05, 2024
Point-of-Need Manufacturing Challenge Demonstrates Technologies for Cold Weather Combat Effectiveness DEC. 15, 2023
Safi Biosolutions rebranding as Safi BiotherapeuticsAUG. 4, 2022
Safi Biosolutions developing alternatives to donated blood as part of USU 4D Bio3 On-Demand Blood programMAR. 24, 2022
iBio Enters into Agreement with Safi Biosolutions to Develop Growth Factors and Cytokines Using the FastPharming® SystemOCT. 2, 2020
nScrypt’s Research Arm Teams Up to Make Human BloodAPR. 7, 2020
Blood Shortage on the Battlefield? Just Make It On-siteFEB. 26, 2020
4D Bio3 Improves Battlefield CareFEB. 27, 2020
Team
Safi Biotherapeutics leadership comprises industry and cell therapy veterans from the Defense Advanced Research Projects Agency (DARPA), Vertex Pharmaceuticals, Mass General Brigham and Loughborough University in the United Kingdom. They bring experience in ex vivo human blood cell development, biologic and pharmaceutical manufacturing, pre-clinical and clinical development, and regulatory submissions.




